(June 29, 2022)
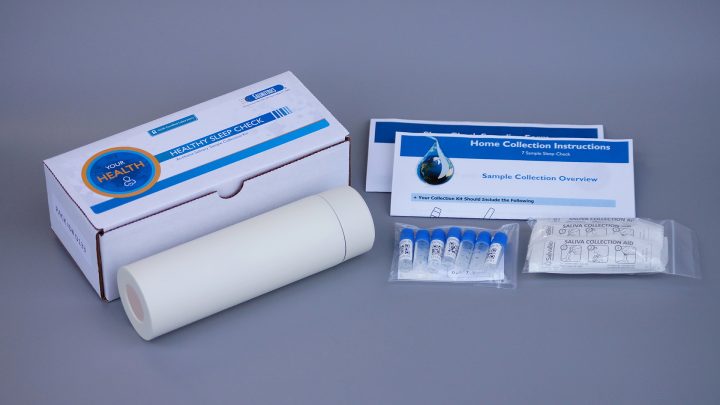
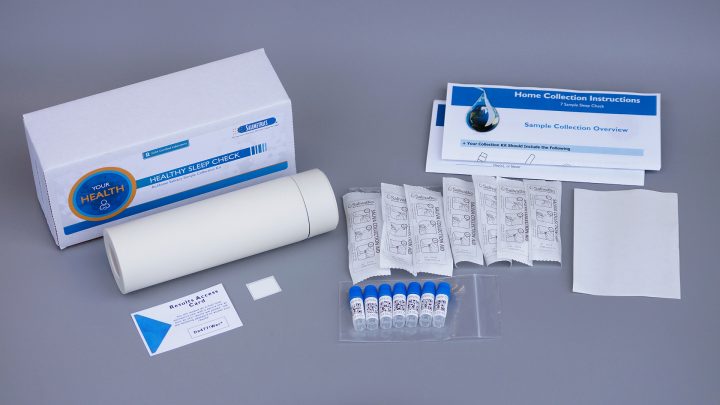
In support of the quest for better sleep, Salimetrics launches a new Circadian Phase Assessment Kit to accurately measure Dim Light Melatonin Onset (DLMO) using saliva. This easy-to-use kit provides a convenient way to accurately measure a patient’s circadian phase with painless sample collection in the convenience of their own home or under supervision at a sleep facility.
Now, sleep healthcare providers have a convenient way to assess the underlying physiology driving their patient’s circadian rhythm with Salimetrics’ Circadian Phase Assessment Kit. “By measuring salivary melatonin levels over time, you are able to determine exactly if and when the body is prepared to go to sleep,” says Steve Granger, Ph.D., Salimetrics Chief Scientific Officer. “Understanding a person’s DLMO can serve as a critical screening tool in diagnosing and treating circadian misalignments as well as other chronic sleep disorders. Determining the timing of the melatonin onset and the amplitude of bedtime melatonin levels complements behavioral sleep phase information and actigraphy for a more comprehensive patient sleep evaluation. Most importantly, Salimetrics has also launched a helpful patient-facing website at http://biologyofsleep.com containing all the necessary background information for patients to learn about DLMO and order their assessment kits directly.”
 Contact: Salimetrics (USA)
Contact: Salimetrics (USA)