Drop Date: March 2020
Shift Right: Assessing Sleep Disorders and Associated Health Concerns Based on Salivary Melatonin and Dim Light Melatonin Onset (DLMO)
In This Article: Shift Right: Assessing Sleep Disorders and Associated Health Concerns Based on Salivary Melatonin and Dim Light Melatonin Onset (DLMO)
With the increased clinical focus on the science of sleep as it relates to human health and performance, salivary melatonin and more specifically, “Dim Light Melatonin Onset” or DLMO, continues to serve as a preferred, reliable tool to assess sleep disorders and associated health concerns.
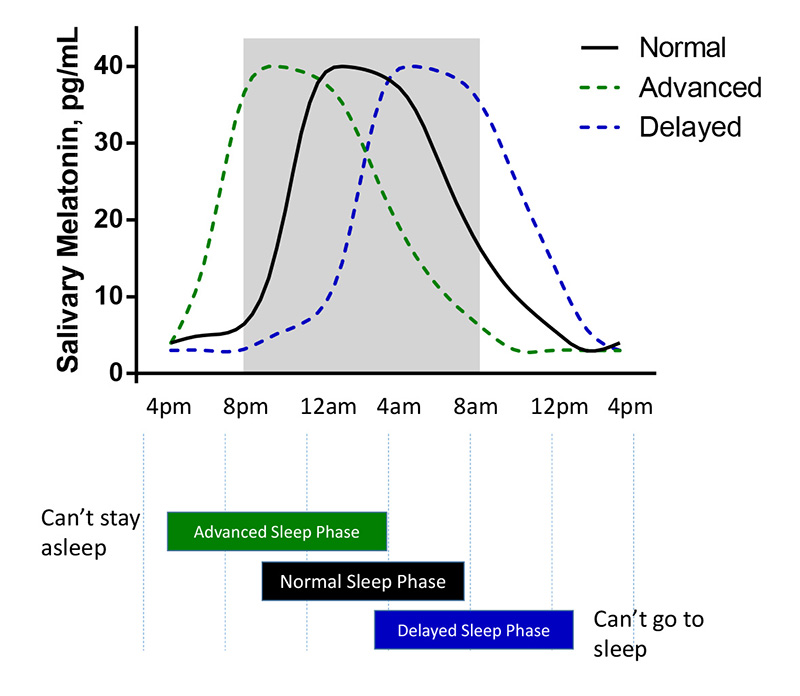
Melatonin (N-acetyl-5-methoxytryptamine) is the sleep-promoting neurohormone and circadian biomarker that serves as a master regulator of circadian rhythm, and measuring DLMO provides the most biologically accurate, evidence-based mechanism to assess circadian phase relative to an individual’s bedtime. A DLMO profile determines the timing of onset production but also importantly shows how much melatonin is in circulation at bedtime. Using saliva provides the opportunity for a non-invasive sample type that does not disrupt a person’s normal sleep cycle, thereby making it the gold standard in circadian rhythm sleep assessment.
Due to the circadian system’s propensity to be modulated by important environmental cues including exposure to bright light, eating, social behavior, exercising, and activity schedules, DLMO patterns have been shown to reliably predict circadian timing and help clinicians more accurately assess sleep behavior and sleep phase shifts that can result in poor concentration, development, productivity, and diminished cognitive performance (Murray et al., 2017), (Burgess et al., 2016; Burgess et al., 2015). Determining DLMO is also relevant in discriminating circadian rhythm related sleep issues from other non-circadian related sleep disorders or establishing the timing of exogenous melatonin administration when treating delayed sleep phase disorders (Lewy et al., 1995).
Salivary Melatonin – The Best Option for DLMO Profiles?
DLMO profiles often require a visit to a sleep lab and are determined by measuring serum melatonin levels over numerous time-points with serial blood draws in patients that have been cannulated. From a patient’s perspective, a preferred DLMO method measures salivary melatonin levels over multiple time points prior to and just after habitual bedtime with samples collected conveniently at home or painlessly in a clinic (Burgess et al., 2016). By utilizing saliva, clinicians have a low-cost solution that significantly improves patient participation. Most importantly, salivary melatonin testing is accurate, reliable, and highly correlated with levels in blood (Nagtegaal et al., 1998). Also, saliva samples can be provided over 7-9 hours, as opposed to an overnight or 24-hour polysomnogram.
Saliva Collection Protocol
Peer-reviewed scientific sleep publication, confirmed by Salimetrics, generally recommends a 7-point sample collection (samples collected every hour beginning 5 hours before normal bedtime, through one hour past bedtime) to provide a reliable DLMO estimation. However, in severely phase-shifted individuals, additional samples may need to be collected over a longer period, as well as in totally blind individuals with a non-24-hour sleep-wake disorder (Keijzer et al., 2014). This protocol was first developed by the Dutch National Referral Center for Sleep-Wake Disturbances and Chronobiology. Samples can be collected with Passive Drool, and typically a small volume of about 0.5 mL is more than sufficient for melatonin measurements.
For advanced precision in calculating DLMO measurements, a 13-point collection (samples collected every half hour, five hours before habitual bedtime, through one hour past bedtime) has also been published in research studies. However, the sample numbers required for this method may be excessive due to increased cost and participant burden. Most studies of this type also concluded that the difference in DLMO estimation was not significant between half-hourly and hourly sampling (Molina and Burgess, 2011). Salimetrics provides collection packs that include either 5 samples or 7 upon request at no additional cost.
Qualified Laboratory: Accurate Results
In practice, accurate and reliable DLMO profiles come from high-quality and experienced laboratories using high quality melatonin assays. Qualified diagnostic and clinical labs adhere to CLIA and GLP regulated standards, and qualified research labs follow NIH requirements for rigor and reproducibility. Finding a lab that adheres to these Seven Attributes of a High-Quality Lab provides confidence in the data received. Clinicians can rely on Salimetrics Clinical Lab for CLIA-Certified testing with HIPAA compliant results reporting, while researchers without access to their own high-quality lab can depend on the Salimetrics’ Saliva Lab – the most qualified and experienced salivary research lab available. Whether performing diagnostic or research testing, the lab should rely on a highly qualified Melatonin Assay Kit as the best choice to ensure accurate and reliable salivary melatonin results.
Salimetrics Melatonin Assay Specifications
To achieve the most reliable and consistent salivary DLMO measures, a highly sensitive and specific salivary melatonin assay is required. The Salimetrics Melatonin Assay is the preferred kit for fast and sensitive measurements of salivary melatonin without the use of radioactivity or needing a time and labor-intensive extraction protocol. Sample measurements using Salimetrics’ Melatonin Assay show leading reproducibility when run in singlicate (low CVs), which provides a high level of confidence in measurements obtained (see example data below).
| Assay Type | Competitive ELISA, colorimetric detection |
| Antibody used | Rabbit Monoclonal Antibody |
| Sensitivity | 1.37 pg/mL |
| Assay Range | 0.78 – 50 pg/mL |
| Assay Time | 3.5 hours |
| Sample Extraction | No |
| Sample Volume | 100 µL per well |
| Number of samples | 38 samples in duplicate, 76 in singlicate |
Interpreting Results
Clinicians and sleep experts routinely calculate DLMO using either the variable threshold method, aka “3k method”, or the fixed threshold method. The fixed threshold method is based on the time at which rising melatonin levels cross a previously determined threshold, typically set at 3 or 4 pg/mL for saliva. This may be applied when baselines are zero. However, this method risks missing DLMO for individuals that are low melatonin producers, a common problem in an aging population. Salimetrics recommends using the 3k method to calculate DLMO (Voultsios et al., 1997, Molina and Burgess, 2011) when baseline samples have measurable levels. This method establishes the mean of the first three low day-time samples and sets the threshold as 2 Standard Deviations above this mean for each person’s own measurements. The 3k method benefits DLMO determinations by including both individuals that are ‘low secretors’ who do not make sufficient melatonin to reach the fixed threshold values and allows for DLMO estimation in individuals who have daytime melatonin levels above the fixed threshold.
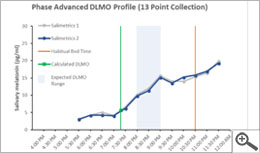
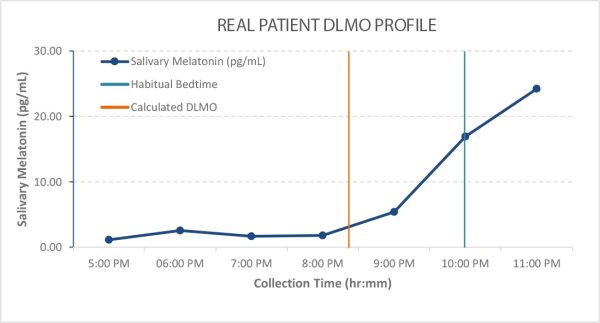
Example DLMO Profile
Data from 7 hourly saliva samples were used to estimate DLMO in the following Phase Response Curve. Using the variable threshold 3k method described above, the time of DLMO was estimated between 8:15-8:30 pm for the following sample profile. Each line in the graph represents one set of singlicate measurements.
Three portions of this DLMO profile provide valuable physiologic information to complement behavioral observations in people with sleep disorders. Baseline melatonin should be very low during daylight hours. Elevated levels during the day are often observed in people who take supplemental melatonin. The time of melatonin onset helps determine if a person has a circadian rhythm sleep disorder. This example shows a normal onset within 2 hours from their routine bedtime. The magnitude of melatonin at bedtime provides insight into whether a person has good sleep hygiene or has been exposed to sufficient daytime light or may need to be evaluated for cataracts. Melatonin is maximally produced when young and slowly declines in amplitude as we age, so a low peak in a young individual is a tell-tale sign of poor sleep hygiene. However, the example above shows a robust peak level and this indicates a younger, healthy individual.
REFERENCES & RELATED RESEARCH
- Burgess, H.J., Park, M., Wyatt, J.K., and Fogg, L.F. (2016). Home dim light melatonin onsets with measures of compliance in delayed sleep phase disorder. Journal of sleep research 25, 314-317.
- Burgess, H.J., Wyatt, J.K., Park, M., and Fogg, L.F. (2015). Home Circadian Phase Assessments with Measures of Compliance Yield Accurate Dim Light Melatonin Onsets. Sleep 38, 889-897.
- Challet, E. (2015). Keeping circadian time with hormones. Diabetes Obes Metab 17 Suppl 1, 76-83.
- Keijzer, H., Smits, M.G., Duffy, J.F., and Curfs, L.M. (2014). Why the dim light melatonin onset (DLMO) should be measured before treatment of patients with circadian rhythm sleep disorders. Sleep Med Rev 18, 333-339.
- Lewy, A.J., Sack, R.L., Blood, M.L., Bauer, V.K., Cutler, N.L., and Thomas, K.H. (1995). Melatonin marks circadian phase position and resets the endogenous circadian pacemaker in humans. Ciba Found Symp 183, 303-317; discussion 317-321.
- Molina, T.A., and Burgess, H.J. (2011). Calculating the dim light melatonin onset: the impact of threshold and sampling rate. Chronobiol Int 28, 714-718.
- Murray, J.M., Sletten, T.L., Magee, M., Gordon, C., Lovato, N., Bartlett, D.J., Kennaway, D.J., Lack, L.C., Grunstein, R.R., Lockley, S.W., et al. (2017). Prevalence of Circadian Misalignment and Its Association With Depressive Symptoms in Delayed Sleep Phase Disorder. Sleep 40.
- Nagtegaal, E., Peeters, T., Swart, W., Smits, M., Kerkhof, G., and van der Meer, G. (1998). Correlation between concentrations of melatonin in saliva and serum in patients with delayed sleep phase syndrome. Ther Drug Monit 20, 181-183.
- Voultsios, A., Kennaway, D.J., and Dawson, D. (1997). Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms 12, 457-466.
- Zelinski, E.L., Deibel, S.H., and McDonald, R.J. (2014). The trouble with circadian clock dysfunction: multiple deleterious effects on the brain and body. Neurosci Biobehav Rev 40, 80-101.
*Note: Salimetrics provides this information for research use only (RUO). Information is not provided to promote off-label use of medical devices. Please consult the full-text article.
 Contact: Salimetrics (USA)
Contact: Salimetrics (USA)